 Deng Xiaoping: 'I have a clear conscience all my life'
Deng Xiaoping: 'I have a clear conscience all my life'
 Xi Jinping: 'The people are our strength'
Xi Jinping: 'The people are our strength'
 Amazing cliff diving in cold winter
Amazing cliff diving in cold winter
 Enjoy Sochi 2014 in slow motion
Enjoy Sochi 2014 in slow motion
 University student sentenced to death for poisoning roommate
University student sentenced to death for poisoning roommate
 Chinese lunar New Year celebrated in San Francisco
Chinese lunar New Year celebrated in San Francisco
 Taiwan Lantern Festival 2014
Taiwan Lantern Festival 2014
 Haiyang Yangge: make up
Haiyang Yangge: make up
 China's top 10 richest cities
China's top 10 richest cities
JERUSALEM, Feb. 24 -- A study led by an Israeli researcher shows that patients suffering from Type 1 diabetes can go for two years without insulin shots, according to a newsletter released by Ben-Gurion University on Monday.
Type 1 diabetes occurs when the body's own immune system destroys the insulin-producing cells, or beta cells, of the pancreas, which are vital in helping the body stay healthy.
Eli Lewis, a doctor and department director at Ben-Gurion University, worked in collaboration with the University of Colorado Health Science Center to study the effects of a new therapy that uses an anti-inflammatory serum protein to control glucose levels in diabetic patients.
Lewis says that following an eight to 12 week treatment, several patients were able maintain proper glucose levels without the need for insulin injections for more than two years.
This is welcome news for people with Type 1 diabetes, many of whom are deficient of the insulin hormone and need regular insulin injections to help the body's immune system fight off foreign invaders like viruses or bacteria.
"This is an excellent beginning in our mission to determine the exciting possibilities of a safe therapy for autoimmune diabetes, and we believe we will see similar results in a number of U.S. patients who recently received this treatment outside the trials within several months of diagnosis and are still completely insulin free," Lewis said.
According to Lewis, the study used an anti-inflammatory serum protein known as Alpha1-Antitrypsin (AAT) to treat the inflammation in injured islets, tiny clusters of insulin-producing cells in the pancreas.
"In addition, diabetes patients, even youngsters and adolescents, showed no adverse affects and a remarkable safety profile," the researcher said.
The treatment course was comprised of a weekly infusion-drip of AAT, then a yearlong follow-up with 12 patients.
The researchers are now recruiting for three more extension trials. However, it will still be a minimum of two years for AAT to receive FDA approval in the United States as an on-label treatment for Type 1 Diabetes.
 Chinese Dream: the Chinese Spirit and the Chinese Way
Chinese Dream: the Chinese Spirit and the Chinese Way 51 bronze sacrificial utensils unearthed in Shaanxi
51 bronze sacrificial utensils unearthed in Shaanxi Most gorgeous female celebs in Chi-pao
Most gorgeous female celebs in Chi-pao Second round of test kicks off at Beijing Film Academy
Second round of test kicks off at Beijing Film Academy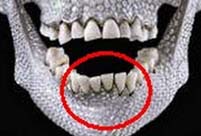 Ancient Qiang people had vertically grown teeth
Ancient Qiang people had vertically grown teeth Top 10 Chinese youth’s favorite seaside destinations
Top 10 Chinese youth’s favorite seaside destinations Traditional Tibetan clothing tailors
Traditional Tibetan clothing tailors In photos: Unveiling Taishan station
In photos: Unveiling Taishan station Beautiful moments of family reunion
Beautiful moments of family reunion Chinese warplanes C919 to appear at Singapore Airshow
Chinese warplanes C919 to appear at Singapore Airshow Ruins of Shang Dynasty's structure unearthed in Shaanxi
Ruins of Shang Dynasty's structure unearthed in Shaanxi  Intercity high speed train in operation
Intercity high speed train in operation Severe coldness freezes large parts of China
Severe coldness freezes large parts of China  Beautiful moments of Sochi
Beautiful moments of Sochi  It's not just performing this year
It's not just performing this yearDay|Week|Month