U.S. advisory panel recommends resuming use of Johnson &Johnson COVID-19 vaccine
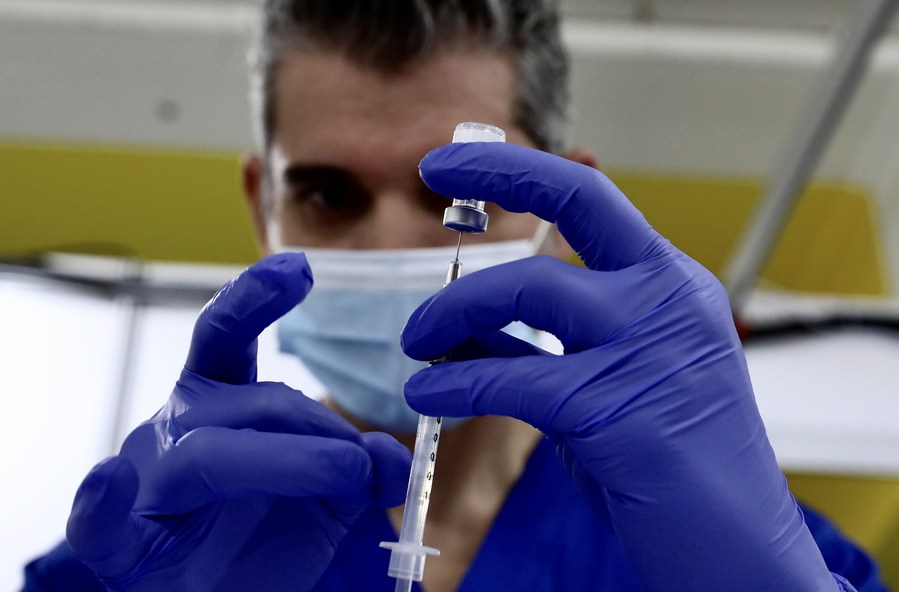
A health care worker prepares a dose of COVID-19 vaccine at a new vaccination site in the California Polytechnic State University in Pomona, Los Angeles County, California, the United States, Feb. 5, 2021. (Xinhua)
Members of the CDC's Advisory Committee on Immunization Practices (ACIP) agreed the benefits of the vaccine outweigh the risks from rare blood clots linked with the vaccine.
WASHINGTON, April 23 (Xinhua) -- An advisory panel to the U.S. Centers for Disease Control and Prevention (CDC) voted Friday to recommend resuming the use of Johnson &Johnson COVID-19 vaccine for adults.
Members of the CDC's Advisory Committee on Immunization Practices (ACIP) agreed the benefits of the vaccine outweigh the risks from rare blood clots linked with the vaccine.
The independent expert panel voted 10 to 4, with one member abstaining, according to media reports.
CDC Director Rochelle Walensky is expected to sign off on the decision and then the U.S. Food and Drug Administration (FDA) will prepare an amended emergency use authorization for the vaccine, Amanda Cohn, ACIP's executive secretary, was quoted by media as saying.
The CDC and the FDA called for a pause in the use of Johnson &Johnson's COVID-19 vaccine on April 13, after six reported U.S. cases of a rare and severe type of blood clot.
In these cases, a type of blood clot called cerebral venous sinus thrombosis was seen in combination with low levels of blood platelets. All the cases occurred among women between the ages of 18 and 48, and symptoms occurred 6 to 13 days after vaccination.
Photos
Related Stories
- U.S. lifts pause on Johnson &Johnson COVID-19 vaccine
- China ready to strengthen vaccine cooperation with Thailand: FM
- U.S. rocked by multiple mass shootings, but major gun legislation unlikely
- Fear of U.S. hate crimes prompts Asians to reconsider study plans: media
- S. Korea's nuke envoy holds phone talks with U.S. counterpart over Korean Peninsula issue
Copyright © 2021 People's Daily Online. All Rights Reserved.