Johnson &Johnson COVID-19 vaccine pause sparks scrambles across U.S., media reports
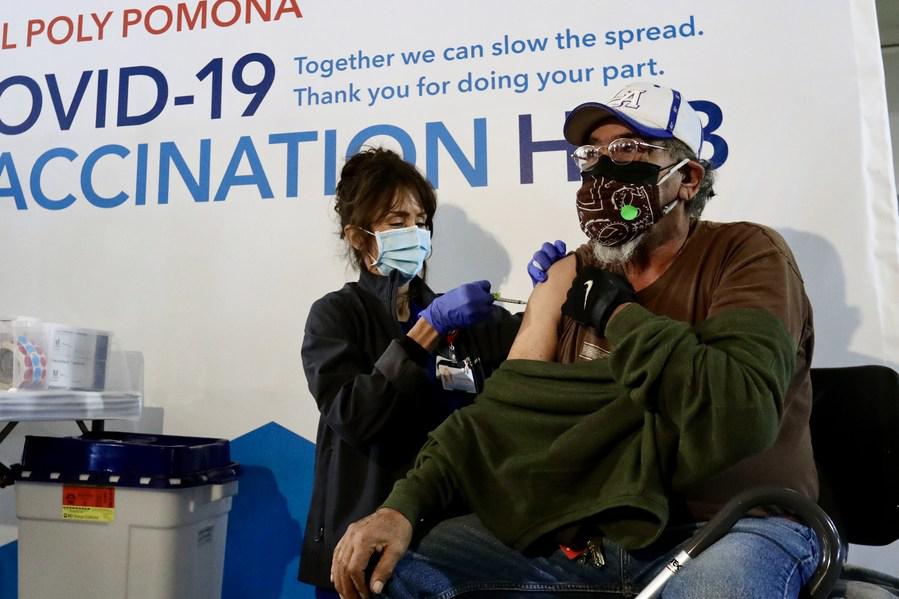
A health care worker administers a dose of COVID-19 vaccine to a recipient at a new vaccination site in the California Polytechnic State University in Pomona, Los Angeles County, California, the United States, Feb. 5, 2021. (Xinhua)
Vaccination sites across the United States have canceled tens of thousands of appointments after health authorities paused the use of Johnson &Johnson's COVID-19 shots. Some researchers said the pause could harden beliefs among the people who believe COVID-19 vaccinations aren't safe, despite their approval by federal health agencies.
NEW YORK, April 15 (Xinhua) -- The pause of use of the Johnson &Johnson COVID-19 vaccine has caused scrambles across the United States as vaccination sites rushed to switch to one of the two other authorized vaccines, according to local media.
Vaccination sites across the United States have canceled tens of thousands of appointments after the country's health authorities paused the use of Johnson &Johnson's COVID-19 shots on Tuesday over reports of rare but severe blood clots, The Wall Street Journal reported on Thursday.
In New York City, 4,000 Johnson &Johnson vaccination appointments were being rescheduled, Mayor Bill de Blasio said on Tuesday. In Alaska, officials said they were holding 24,322 doses of the Johnson &Johnson vaccine that had not yet been administered pending further review. And in Washington state, hundreds of patients scheduled to receive the single-dose vaccine at a fairground were told they would instead get the first of two doses of the vaccine developed by Pfizer Inc. and BioNTech SE.
Many more states announced that they would follow the new guidelines from the Food and Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC), said the report.
Nationwide, federal officials expected to allocate about 26.5 million doses of the Pfizer-BioNTech and Moderna Inc. vaccines combined this week, compared with 1.5 million of Johnson &Johnson's.


People line up to enter a mass COVID-19 vaccination site at the United Center in Chicago, the United States, on March 10, 2021. (Photo by Joel Lerner/Xinhua)
Federal authorities recommended a temporary halt to use of the Johnson &Johnson vaccine after six women between the ages of 18 and 48 years who got the vaccine developed blood clots, and one died.
Over seven million doses of the vaccine have been administered in the United States, according to the CDC. Johnson &Johnson's vaccine was the third to be approved after those of Pfizer-BioNTech and Moderna. Unlike the other two, it requires only one dose to take effect.
Johnson &Johnson's single dose has emerged as the preferred vaccine for some underserved communities, including the homeless and certain isolated communities where patients find it harder to return for a second injection, according to the Journal.
Some remote rural counties in the United States were already contending with a shortage of qualified medical personnel to administer vaccines, or a lack of chain pharmacies with proper infrastructure to distribute jabs.
Some health researchers said the pause could harden beliefs among the people who believe that COVID-19 vaccinations aren't safe, despite their approval by federal health agencies.
A February poll by the Pew Research Center found that 30 percent of Americans said they don't plan on getting vaccinated, with the majority citing side effects as their main concern.
Photos
Related Stories
- U.S. regional manufacturing continues to improve in April: survey
- U.S. agricultural futures close mixed
- Johnson &Johnson's COVID-19 vaccine pauses pending investigation as public trust plummets
- U.S. weekly jobless claims drop to 576,000, lowest since pandemic outbreak
- U.S. Senate votes to open debate on anti-Asian American hate crimes bill
Copyright © 2021 People's Daily Online. All Rights Reserved.